In materials science and engineering, the concept of a spatially varying function arises frequently:
There are also spatially variable intensive quantities:
Each example is a scalar function of space--that is, the function associates a scalar with each point in space.
A topographical map is a familiar example of a graphical illustration of a scalar function (altitude) as a function of location (latitude and longitude).
However, there are many other types of scalar functions of
several arguments, such as the state function:
![]() or
or
![]() .
It is sometimes useful to think of these types of functions a scalar
functions of a ``point'' in a thermodynamics space.
.
It is sometimes useful to think of these types of functions a scalar
functions of a ``point'' in a thermodynamics space.
However, this is often a source of confusion: notice that
the internal energy is used in two different contexts above.
One context is the value of the energy, say 128.2 Joules.
The other context is the function
![]() .
While the two symbols are identical, their meanings are quite different.
.
While the two symbols are identical, their meanings are quite different.
The root of the confusion lurks in the question, ``What are the
variables of ![]() ?''
Suppose that there is only one (independent) chemical species, then
?''
Suppose that there is only one (independent) chemical species, then ![]() has
three variables, such as
has
three variables, such as ![]() .
``But what if
.
``But what if
![]() ,
,
![]() , and
, and
![]() are known functions, what are the variables
of
are known functions, what are the variables
of ![]() ?''
The answer is, they are any three independent variables, one
could write
?''
The answer is, they are any three independent variables, one
could write
![]() and there are six other natural choices.
and there are six other natural choices.
The trouble arises when variations of a function like ![]() are
queried--then the variables that are varying must be
specified.
are
queried--then the variables that are varying must be
specified.
For this reason, it is either a good idea to keep the functional form explicit in thermodynamics, i.e.,
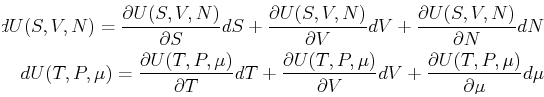 |
(12-6) |
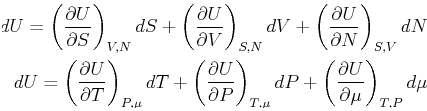 |
(12-7) |