 |
A useful graphical construction can be utilized for extracting values of chemical potentials:
Consider that the molar free energy of a solution is plotted as follows:
 |
It would be particularly useful to obtain the chemical potentials of each species in solution as a function of composition. This relationship can be determined as follows:
Starting with an expression for the molar free energy of the solution being a weighted sum of the chemical potentials:
| (28-5) |
![]() becomes when using
becomes when using
![]() .
.
or
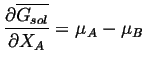 |
(28-6) |
at constant
![]() and
and
![]() ; similarly,
; similarly,
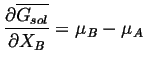 |
(28-7) |
Multiplying
![]() by
by
![]() and subtracting
it from
and subtracting
it from
![]() :
:
 |
(28-8) |
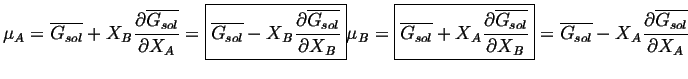 |
(28-9) |
These equations can be interpreted with the following figure.
 |