What happens when too much salt is added to a solution?
Clearly, a solubility limit must exist and a new phase will appear.
This can be qualitatively understood by considering the
behavior of the molar Gibbs free energy of forming
a solution as a function of the amount of solute
![]() :
:
To quantify the conditions for the appearance of new phases, consider the thermodynamics of binary (i.e. two component) alloys1
The Gibbs-Duhem equation of a system
consisting of two components
![]() and
and
![]() is:
is:
Consider a closed system consisting of one mole of molecules:
![]() mole:
mole:
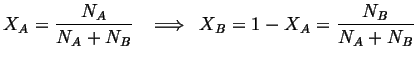 |
(28-1) |
The state of the system (per mole) should be representable by
three independent parameters,
![]() or
or
![]() .
Therefore, using
.
Therefore, using
![]() and
and
![]() in the Gibbs-Duhem equation:
in the Gibbs-Duhem equation:
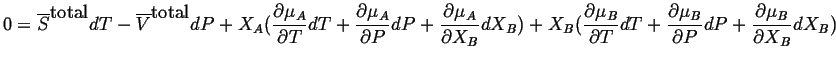 |
(28-2) |
where
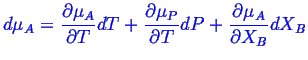
Note that
![]() and
and
![]() ,
thus,
,
thus,
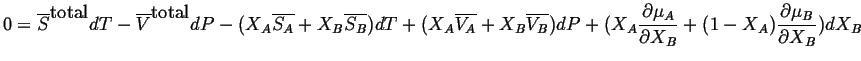 |
(28-3) |
The first and third terms cancel and the second and fourth terms cancel. Therefore,
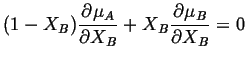 |
(28-4) |
This is a general result for binary solution. It is the form of the Gibbs-Duhem equation for solutions. Equation 28-4 gives a relation between the derivatives of the chemical potentials but not a relation between the chemical potentials themselves.