Question: What are the units of
![]() ? What are the units of
? What are the units of
![]() ?
?
Imagine that there is a completely homogeneous system that has been arbitrarilry
divided into small volumes of size
![]() :
:
 |
Consider what is left of the internal energy after we subtract off the ``mechanical'' or ``compression'' energy:
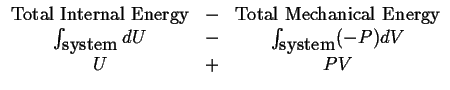 |
(11-12) |
So that we can define a new state function (that is also extensive),
| (11-13) |
It is sensible to interpret
![]() as the ``thermal
energy'' at constant pressure.
In other words, we divide the internal energy for a simple fluid
as the ``thermal
energy'' at constant pressure.
In other words, we divide the internal energy for a simple fluid
![]() into two parts--one part corresponding to that stored as
compressive energy (
into two parts--one part corresponding to that stored as
compressive energy (
![]() ) and another part the thermal energy
) and another part the thermal energy
![]() .
Another way to see this is:
.
Another way to see this is:
| (11-14) |
In other words
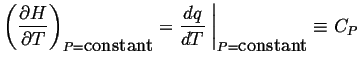 |
(11-15) |
for an ideal gas:
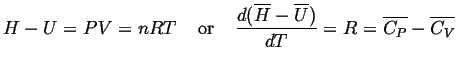 |
(11-16) |