Recall that
![]() is not a perfect
differential.
is not a perfect
differential.
Let's consider
![]() for an ideal gas
undergoing a reversible process.
for an ideal gas
undergoing a reversible process.
| (11-17) |
for an ideal gas
| (11-18) |
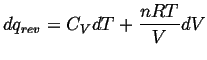 |
(11-19) |
Now divide through by
![]()
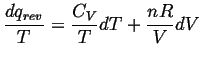 |
(11-20) |
Notice that we have separated the equation
into something that is integrable over
segments of
![]() and
and
![]() and thus over any
curve.
and thus over any
curve.
Therefore,
![]() is a ``perfect
differential'' and it must then be
a state function for an ideal gas.
is a ``perfect
differential'' and it must then be
a state function for an ideal gas.