Consider two bodies each with the same volume
![]() and the same pressure. Bring the bodies
together, what is the result?
and the same pressure. Bring the bodies
together, what is the result?
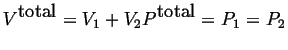 |
(03-2) |
Variables that are additive like
![]() are
called extensive variables, they depend
on the size or the extent of the system.
are
called extensive variables, they depend
on the size or the extent of the system.
Variables that do no add, but combine like
![]() are
called intensive variables, they are
intrinsic to the system and can vary from
point to point. Fields and
potentials are intensive variables.
are
called intensive variables, they are
intrinsic to the system and can vary from
point to point. Fields and
potentials are intensive variables.
Another example: Temperature:2
Question: Is
![]() an extensive or intensive
variable?
an extensive or intensive
variable?
Can you design a small device that would measure the temperature at a point? (what does it resist changes in?)
This is a difficult question because we haven't defined temperature.
Leads to difficult argument found in thermodyanamics books (e.g., Denbigh §1.4) which can be extended to an even more difficult argument that defines the absolute zero of temperature.