Question: What kinds of variables describe a material body and the changes that occur as it is manipulated
How does one describe a body or a system completely? There are at least two plausible approaches:
Method of description: 1
Describe for each particle in the body
Question: Why is Avogadro's number,
![]() so big?
so big?
Keeping track of
![]() molecules is just
not humanly (or even, in this decade at least, computerly)
possible.
molecules is just
not humanly (or even, in this decade at least, computerly)
possible.
Method of description: 2
Determine a quantity that reflects the average or sum total properties of the body
Examples:
Question: Can you identify others?
Question: If an applied force,
![]() , tries to make
a spring get shorter (larger opposing forces
, tries to make
a spring get shorter (larger opposing forces
![]() shorter spring)--what is it that
makes the volume of a body get smaller?
shorter spring)--what is it that
makes the volume of a body get smaller?
How would you measure pressure?
Design a small gauge:
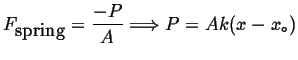 |
(03-1) |
Compression, caused by large positive pressures, compresses the spring. In other words, a negative force in a spring is compressive; thus pressure and force have different sign conventions. Tensile forces extend the spring and are associated with negative pressures.1
What kinds of things might be wrong with our little device to cause it to give
bad measurements of pressure?
Imagine that we made our device very small.
In the limit of an infinitely small device, we could use it to measure pressure from point to
point:
![]() .
.
Question: Practically, how small would be too small?
Question: Would two bodies be in equilibrium if they were in mechanical contact and their pressures are different?
Question: Are pressure and volume similar variables? How are they naturally coupled?