Let's just state the zeroeth Law of Thermodynamics
If bodies
![]() and
and
![]() are in thermal but not mechanical
contact and heat flows from
are in thermal but not mechanical
contact and heat flows from
![]() to
to
![]() and if
and if
![]() is in
thermal contact with
is in
thermal contact with
![]() and heat flows from
and heat flows from
![]() to
to
![]() then
then
Heat will flow from
![]() to
to
![]() if they are in thermal
contact. In other words,
if they are in thermal
contact. In other words,
| (03-3) |
| (03-4) |
We can call the body
![]() a thermometer and use the
zeroeth law.3
a thermometer and use the
zeroeth law.3
The temperature can be related to
![]() , we can
just call
, we can
just call
![]() . This can be done rigorously
and it defines an absolute zero (
. This can be done rigorously
and it defines an absolute zero (
![]() )
but let's not do this and move on.
)
but let's not do this and move on.
Later we may use the entropy,
![]() and internal
energy,
and internal
energy,
![]() , of a body to define the
temperature.4
, of a body to define the
temperature.4
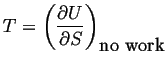 |
(03-5) |
For a body that does no work, or has no work performed on it, the temperature is the rate at which internal energy changes with entropy.
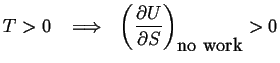 |
(03-6) |
![]() tends to increase with
tends to increase with
![]() .
.
In this lecture, we have discussed state variable that gives us information about a body, an efficient way.

All of these quantities rely on measurement but are assumed to be independent of the type of measurement.