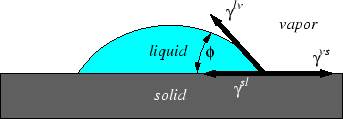Next: Defect Equilibria
Up: Continuum Thermodynamics
Previous: Electrochemistry
A material can store energy at interfaces. The energy contribution is the surface tension
multiplied by the area of interface. Surface tension can result in the pressure of two
phase being different if the interface between them is curved. The chemical potential
at an interface is increased if it is curved, this is the Gibbs-Thompson effect.
Material can segregate preferentially to interfaces if it decreases the surface tension.
This follows from the Gibbs-Duhem equation for surfaces and the result is called the Gibbs Absorption
equation.
Surface tensions act a force balances and specify the contact angle between two phases:
Figure 2-7:
Young's Equation for a flat surface
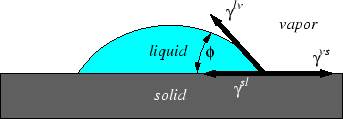 |
Considerations of the geometry of such surfaces has important implications on
the sizes of nuclei and therefore on the entire topic of phase transformations.
W. Craig Carter
2002-09-05