| (32-10) |
Above, a ``first-order'' correction to the ideal solution model based on an atomic averaging for the enthalpy of mixing.
This is called the regular solution model.
| (32-10) |
where
 |
(32-11) |
and
| (32-12) |
Consider both terms:
Note that
![]() favors mixing, and makes sense
because
favors mixing, and makes sense
because
![]() is more negative than
is more negative than
![]() .
.
So that, taken together:
![]() :
:
Note that the limiting behavior for pure or extremely dilute solutions is dictated by:
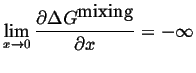 |
(32-13) |
A solution can always lower its free energy by dissolving at least a small amount component. There is thermodynamically always some finite solubility (but it can be, and often is, very, very small). This implies that the width of a single-phase region must always be finite.
Consider the case where
![]() , so the
system will tend to ``unmix'' at low
temperatures.
, so the
system will tend to ``unmix'' at low
temperatures.
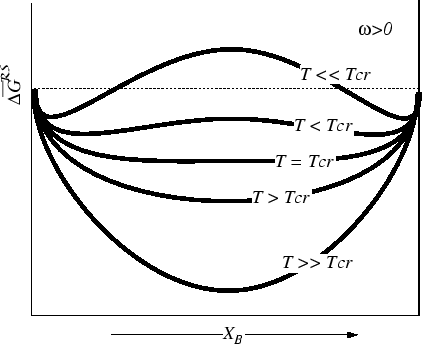 |
For the case of a regular solution,
the curve is symmetric around
![]() , and in this case we can
calculate the positions of the common tangents:2
, and in this case we can
calculate the positions of the common tangents:2
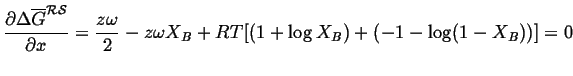 |
(32-14) |
The critical temperate can be determined analytically by noting
that as the common tangents form that the curvature changes
sign at
![]() .
.
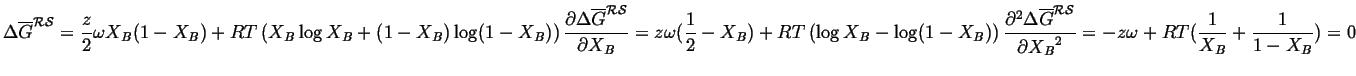 |
(32-15) |
At
![]() , the zero first appears at
, the zero first appears at
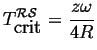 |
(32-16) |