In this section, a simple model for the enthalpy of mixing will be derived. It will be shown that a positive enthalpy of mixing tends to make a system separate and have a miscibility gap at low temperature. A negative enthalpy of mixing tends to favor stable homogeneous solutions.
Consider two neighbors in a solution. The
probability that one of the neighbors is an
![]() -type or a
-type or a
![]() -type is simply:
-type is simply:
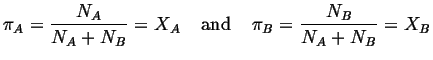 |
(32-1) |
Therefore the probability that a given
``bond'' is an
![]() -
-
![]() type is:
type is:
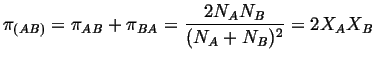 |
(32-2) |
If each atom has
![]() nearest neighbors, the
number of bonds, total, is
nearest neighbors, the
number of bonds, total, is
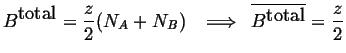 |
(32-3) |
The bond density of
![]() -
-
![]() type is therefore:
type is therefore:
| (32-4) |
If the energy per
![]() bond is
bond is
![]() 1then the enthalpy density (due to the
1then the enthalpy density (due to the
![]() -
-
![]() bonds) is:
bonds) is:
| (32-5) |
Similarly, the bond density of
![]() -types is
-types is
![]() so that
so that
![]() .
Similarly
.
Similarly
![]() .
.
Putting this all together
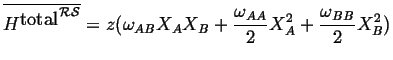 |
(32-6) |
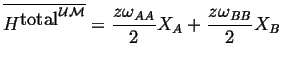 |
(32-7) |
Therefore since,
![]() :
:
 |
(32-8) |
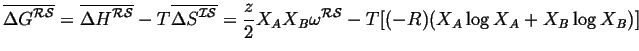 |
(32-9) |
This is the Regular Solution Model