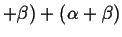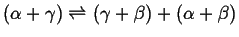

Next: About this document ...
Up: Lecture_30_web
Previous: A Menagerie of Binary
There are two fundamental ways that invariant points can arise:1
- When two two-phase regions join at a temperature
and become one two-phase region:
- Eutectic
-
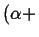 liquid
liquid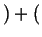 liquid
liquid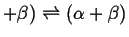
- Eutectoid
-
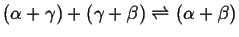
Figure:
Eutectic-type (EV-TYPE at MASSACHVSETTS
INSTITVTE OF TECHNOLOGY) invariant points.
 |
- When one two-phase region splits into two two-phase
regions:
- Peritectic
-
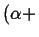 liquid
liquid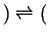 liquid
liquid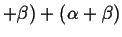
- Peritectoid
-
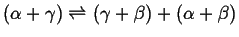
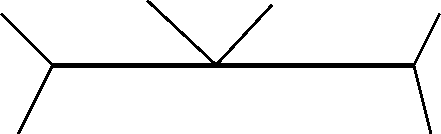
Figure 30-15:
Peritic-type invariant points.
 |
The invariant points determine the topology of the phase diagram:
Figure 30-16:
Construct the rest of the Eutectic-type phase diagram by
connecting the lines to the appropriate melting points.
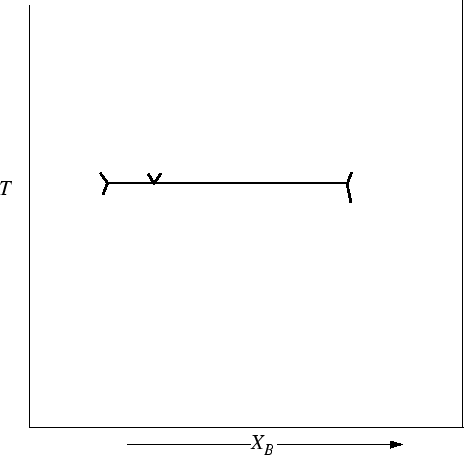 |
Figure 30-17:
Construct the rest of Peritectic-type phase diagram, on the
left a rule for all phase diagrams is illustrated--the ``lines'' must metastably ``stick''
into the opposite two phase region.
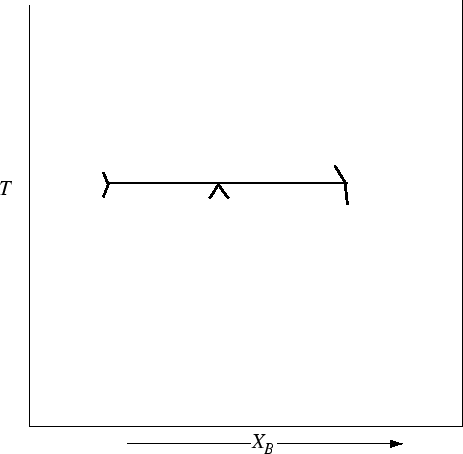 |
These diagrams can be combined and drawn:
Figure 30-18:
Construct the lens over peritectoid phase diagram.
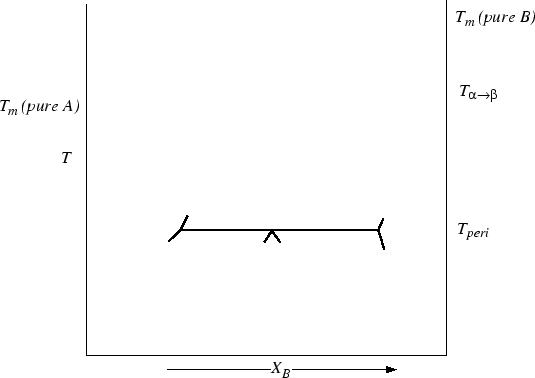 |
Figure 30-19:
Construct the peritectic over eutectic phase diagram.
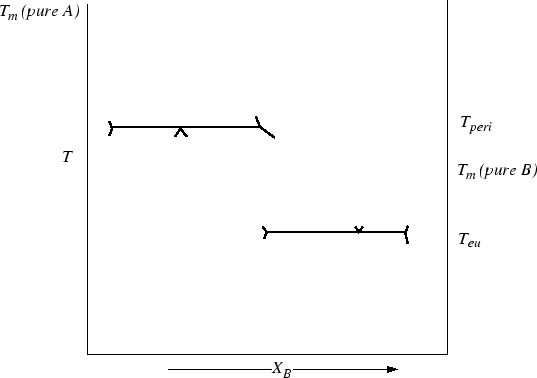 |
In all cases, you should be able to predict how the phase fractions and
equilibrium compositions change as you reduce the temperature at equilibrium.


Next: About this document ...
Up: Lecture_30_web
Previous: A Menagerie of Binary
W. Craig Carter
2002-12-03
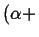 liquid
liquid liquid
liquid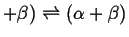
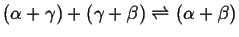
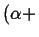 liquid
liquid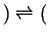 liquid
liquid