| (20-24) |
Let's consider a more complicated gaseous reaction:
| (20-24) |
Suppose we have a container at 1 atm and at 1000
![]() C with initially
two moles of water vapor and one mole of carbon dioxide.
C with initially
two moles of water vapor and one mole of carbon dioxide.
Here are some questions that one might ask:
How many moles of oxygen gas will exist at equilibrium?
If the reaction is carried out at a different pressure, how will the concentration of carbon monoxide vary?
The first question may seem a little odd--there appears to be no oxygen in the reaction 20-24.
However, if reaction 20-24 is written as the sum of two reactions:
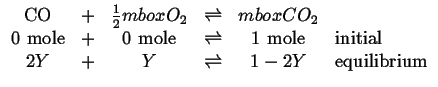 |
(20-25) |
and
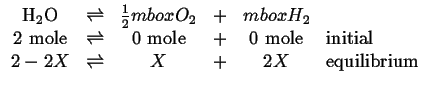 |
(20-26) |
Then it is clear that oxygen plays a role in the reaction 20-24 as the there are two independent reactions contained in the three reactions 20-24, 20-25. and 20-26.
Furthermore, the sum of the standard free energies for the reaction will also add like the reactions and each of the reactions will have a standard reaction and the dependent reaction will be related to the others:
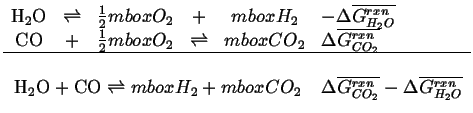 |
(20-27) |
The total number of moles of ideal gas in the system at equilibrium will be
![]() .
(n.b., the reaction 20-25 produces
.
(n.b., the reaction 20-25 produces
![]() moles and the reaction 20-26
prooduces
moles and the reaction 20-26
prooduces
![]() moles in addition to the initial 3 moles.)
Every independent reaction must be at equilibrium independently
Therefore we can write the two equilibrium conditions for the reaction 20-25
moles in addition to the initial 3 moles.)
Every independent reaction must be at equilibrium independently
Therefore we can write the two equilibrium conditions for the reaction 20-25
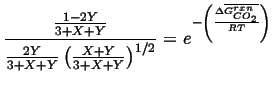 |
(20-28) |
and for reaction 20-26,
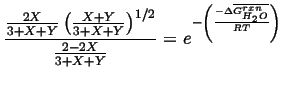 |
(20-29) |
so there are two independent equations and two unknowns, so it is now algebra to
find
![]() and
and
![]() and therefore the concentration of oxygen
and therefore the concentration of oxygen
![]() .
.
To find how the concentration of carbon monoxide depends on pressure, rewrite Eq. 20-28 in terms of partial pressures:
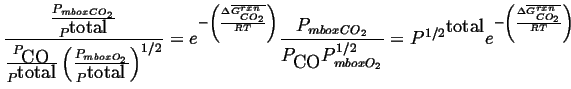 |
(20-30) |