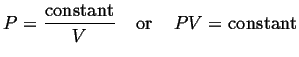 |
(10-3) |
Experimental Observations:
Boyle's Law: At constant temperature. The pressure of a fixed amount of a gas varies inversely with its volume.
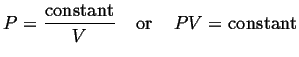 |
(10-3) |
Question: How would you show this experimentally?
Charle's Law (or Gay-Lussac)
The constant from Boyle's Law varies approximately linearly with the temperature of the gas.
| (10-4) |
from which follows (since
![]() is extensive):
is extensive):
| (10-5) |
Traditionally,
![]() is used when
is used when
![]() is the number
of moles, but we could define it as the
molar version of Boltzmann's constant:
is the number
of moles, but we could define it as the
molar version of Boltzmann's constant:
![]() .
.
For a general, (nonpolar, non-magnetic, non-anything-else-that-can-store-work) fluid, we can write a state function
| (10-6) |
The ideal gas is just one example of such a function:
| (10-7) |
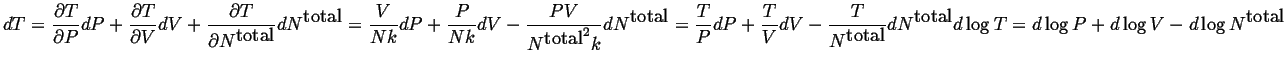 |
(10-8) |
An ideal gas can be thought of a set of point particles that have no finite range forces interactions-this is clearly an idealization, since we know that molecules take up a finite amount of space and that forces can exist between gas molecules.
On closer examination of experimental data, it is found that gasses only approximate this behavior; van der Waals added a few extra constants to fit the data better:
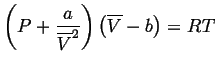 |
(10-9) |
When
![]() , Equation 10-9 is the same as Equation 10-5.
, Equation 10-9 is the same as Equation 10-5.
![]() accounts the finite size of the molecule.
accounts the finite size of the molecule.
![]() is a measure of forces between
molecules thus affecting pressure.
is a measure of forces between
molecules thus affecting pressure.
Both Equation 10-9 and Equation 10-5 are models for material behavior. They are independent of thermodynamic principles, but the behavior of any material is subject to the laws of thermodynamics. So, thermodynamics limits the types of equations that can be used as state functions for material behavior.