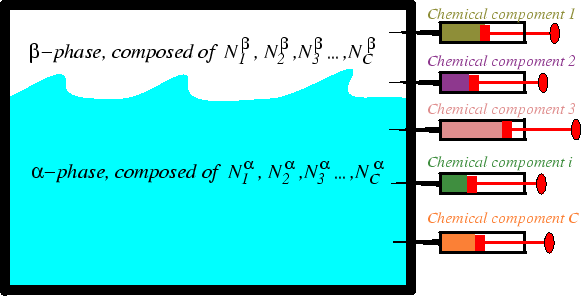 |
If we consider an open chemical system, then we can change the internal energy of a body by alterations of the composition.
For instance, we know that salt has a maximum solubility in water (it is about 300 g/l). If a salt crystal is placed in contact of an open system of water of fixed volume, then chemical work is performed by the dissolution of the salt crystal (or precipitation of salt if the water system is supersaturated).
We will discuss chemical work at length later in the course--but it may be useful to ask yourself the following:
Question: What is the natural ``force'' against which one must to work to add molecules to a system? Should this force depend on the type of molecule being added to the system? Should the value of the force depend on the chemical composition of the system to which the molecules are being added?
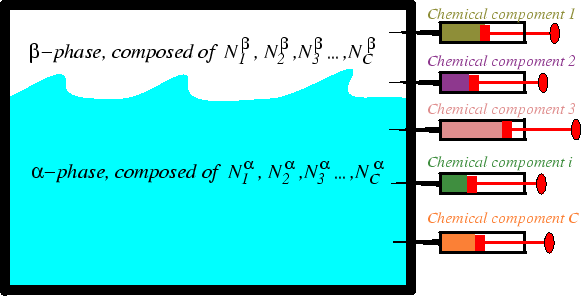 |
It may be useful to think of a particular case. Consider a regular kitchen sponge that has been dried out in the oven for a few days and then exposed to a humid environment. Is there a force driving water molecules from the vapor into the sponge? When does the force disappear? Consider a sponge that has been soaking in liquid water and then placed in the desert--is there a force on the water molecules driving them one way or another?