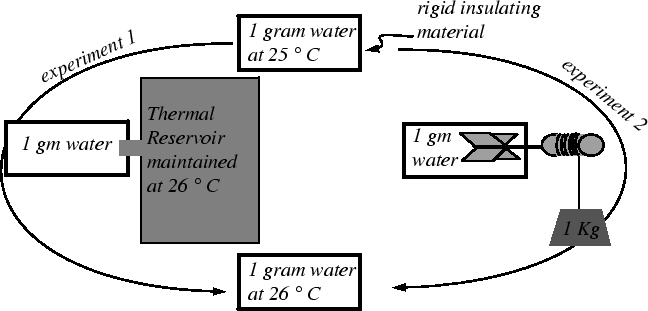
 |
Discussion: Name some ways that one can heat up (i.e. increase the temperature of) a fixed mass of Pb.
The observation that heat and work can independently produce the exact same changes on a body is obvious today. I can't imagine what it is like not to understand this intuitively.
The classical way of explaining this is the following:
I've abstracted the following story about James Joule and the introduction of the first law of thermodynamics from Bent's book.
 |
James Joule is credited with the first careful experiments and
analysis that led us to our present understanding of the first law.
Joule worked in his family's brewery in Manchester, England and
at the age of nineteen in 1838
became interested in the improvement of electric motors.
He discovered by various means that he could heat a body of water
by purely mechanical means: a) by lowering a weight and
letting a paddle wheel stir the water; b) by passing electric current
through a resistor; c) by compressing a piston immersed in the water;
d) by friction from rubbing blocks together. He found that about 800
foot-pounds (
![]() 1 kilojoule) of work could raise the temperature of one
pound (.45 kilograms) of water one Fahrenheit degree (0.55
1 kilojoule) of work could raise the temperature of one
pound (.45 kilograms) of water one Fahrenheit degree (0.55
![]() C).
The result was presented to the British Association in 1843 (Joule was
then 24). It was received with
``entire incredulity'' and ``general silence.''
C).
The result was presented to the British Association in 1843 (Joule was
then 24). It was received with
``entire incredulity'' and ``general silence.''
In 1844, a paper on the subject was rejected by the Royal Society.
Joule must have had a combination of great confidence in his conclusions, extraordinary temerity, and will. In 1845, he discussed his results and suggested that water at the bottom of a waterfall should have a slight temperature increase from water at the top of a waterfall. He also suggested that data from the thermal expansion of gases indicated that there was a lowest possible temperature that could be achieved--this was the first suggestion of absolute zero on the temperature scale. These results failed to provoke discussion.
Two years passed and once more in 1847, Joule presented his maturing work at an Oxford meeting of the British Association. As Joule later remarked, ``... the communication would have [again] passed without comment if a young man has not risen in the section, and by his intelligent observations created a lively interest in the new theory. The young man was William Thomson [later Lord Kelvin] who had two years previously passed at the University of Cambridge with the highest honour, and is now [1885] probably the foremost scientific authority of the age.''
In later years Thomson recalled that he was ``tremendously struck'' by Joule's paper, and added, "This is one of the most valuable recollections of my life, and is indeed as valuable a recollection as I can conceive in the possession of any man interested in science.''
Thomson has given this account of the historic 1847 Oxford meeting. `` ...as I listened [to Joule's paper] I saw that [he] had certainly a great truth and a great discovery, and a most important measurement to bring forward....
``...Faraday was there and was much struck with it, but did not enter fully into the new views. It was many years after that before any of the scientific chiefs began to give their adhesion.''
``... Miller and Graham, or both, we for many year quite incredulous as to Joule's results, because they all depended on fractions of a degree of temperature, sometimes very small fractions. His boldness in making such large conclusions from such very small observational effects is almost as note-worthy and admirable as his skill in extorting accuracy from them. I remember distinctly at the Royal Society, I think it was either Miller or Graham saying simply he did not believe in Joule because he had nothing but a hundreths of a degree to prove his case by''
In the same account, Thomson adds that soon after the Oxford meeting he was walking near Chamonix at the beginning of a hike at Mt. Blanc in the French Alps, ``and whom should I meet walking up but Joule, with a long thermometer in his hand, and a carriage with a lady in it not far off. He told me that he had been married since we had parted at Oxford! and he was going to try for the elevation of temperatures in waterfalls.''
Joule's paper ``On the Mechanical Equivalent of Heat'' was communicated by Faraday to the Royal Society in 1849 and appeared in Philosophical Transactions in 1850. The last paragraph of this historic paper ends with the statements:
I will therefore conclude by considering it as demonstrated by the experiments contained in this paper:
A third proposition, suppressed by the publication committee, state that friction consists of a conversion between mechanical work into heat.