Pressure could be a state function of the variables,
![]() the number
of atoms present,
the number
of atoms present,
![]() the volume of the container, and
the volume of the container, and
![]() the
temperature:
the
temperature:
| (04-1) |
Note that the word pressure plays two roles here!
One role is as a thermodynamic variable that could
be measured and the other is the name of some function.
Another way to think of this is that for an arbitrary
system, there is no reason to expect that there might be
a function called pressure that relates to only
![]() ,
,
![]() , and
, and
![]() but there is nothing preventing us from measuring the
pressure anyways.
However, if we are considering an ideal gas model for our
system, then there is a function
but there is nothing preventing us from measuring the
pressure anyways.
However, if we are considering an ideal gas model for our
system, then there is a function
![]() which tells
us that we need not bother to measure
which tells
us that we need not bother to measure
![]() if we know
if we know
![]() ,
,
![]() , and
, and
![]() --or
we can use our knowledge of
--or
we can use our knowledge of
![]() for an ideal gas to construct
a pressure gauge.
for an ideal gas to construct
a pressure gauge.
Another state function is total number of molecules or atoms:
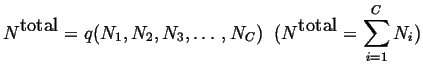 |
(04-2) |
Total mass:
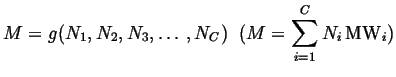 |
(04-3) |