


Next: Le Chatelier's Principle
Up: Continuum Thermodynamics
Previous: Chemical Reaction Equilibria
There are some mathematical consequences of our friends,
 ,
,
 ,
,
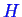 ,
,
 , and
, and
 , being state
functions. They are the Maxwell relations. It is useful to know they exist because it allows
you to form relations between different quantities that are not intuitive at all. They are
good fodder from questions on exams in other thermodynamics courses. I'd like you to understand
where they come from and be able to manipulate them in the calm quiet atmosphere of your home,
but I won't put questions about them on exams. What's the point?
I would feel perfectly comfortable asking you questions about why they are
important or where the Maxwell relations come from.
There are also some implications that follow from the maths that imply how you should
draw free energy curves--for some reason, materials scientists are amused by this fact.
, being state
functions. They are the Maxwell relations. It is useful to know they exist because it allows
you to form relations between different quantities that are not intuitive at all. They are
good fodder from questions on exams in other thermodynamics courses. I'd like you to understand
where they come from and be able to manipulate them in the calm quiet atmosphere of your home,
but I won't put questions about them on exams. What's the point?
I would feel perfectly comfortable asking you questions about why they are
important or where the Maxwell relations come from.
There are also some implications that follow from the maths that imply how you should
draw free energy curves--for some reason, materials scientists are amused by this fact.
We will have to go through some exercises about how to do mathematical manipulations in
thermodynamics. I happen to like this kind of thing. You are expected to have
seen them so I will teach them to you and give you some homework exercises.



Next: Le Chatelier's Principle
Up: Continuum Thermodynamics
Previous: Chemical Reaction Equilibria
W. Craig Carter
2002-09-05