


Next: Mathematical Thermodynamics
Up: Continuum Thermodynamics
Previous: Gibbs Free Energy
One immediate consequence of Gibbs free energy being minimized is that we can calculate the
equilibrium concentrations in a chemical reaction from the molar change in Gibbs free energy
of the reaction. The resulting equation is a fraction containing chemical activities raised
to stoichiometric powers is equal to an equilibrium constant that can be calculated from
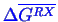 .
.
This minimization leads directly to what may be a familiar formula for the
equilibrium concentrations in a chemical reaction, i.e.,
 |
(02-2) |
W. Craig Carter
2002-09-05

