Last time: Spinodal decomposition--III; Nucleation--I.
Spinodal microstructures
Later stages
Nucleation theory: Introductory concepts
Today: Nucleation--II.
Role of elastic strain energy in nucleation
Theory for steady-state nucleation rate
Heterogeneous Nucleation
Nucleation theory--II.
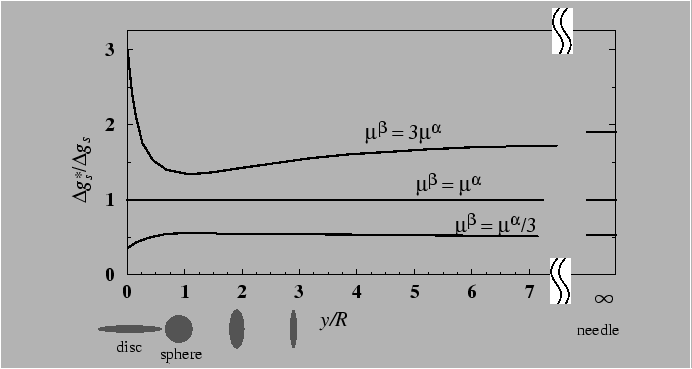
Role of elastic strain energy
In supersaturated crystalline solid solutions the first particles of the new phase that form are generally coherent with the matrix, because of the lower interphase boundary energy relative to semicoherent or incoherent interfaces. However, coherent inclusions have an associated elastic strain energy that resists nucleation. This elastic strain energy is not easy to express with simple algegraic expressions, except for particularly ideal cases.
If nucleation of an incoherent particle were to occur, it
would do so without attendant elastic strain energy, because the
incoherent interface would act as a perfect source or sink of point
defects and the successful critical nucleus, with the lowest ![]() , would be a configuration without the energy penalty of elastic
strain energy.
, would be a configuration without the energy penalty of elastic
strain energy.
The method for calculating the elastic strain energy of coherent inclusions was developed most elegantly by J.D. Eshelby, and it uses an idealized procedure to create the inclusion and calculate the accompanying strain energy. Eshelby's procedure consists of four steps:
The easiest class of problems of this type to solve make these
assumptions: elastic isotropy, elastic homogeneity (same elastic
constants in particle and matrix), and that the particle shape is an
ellipsoid of revolution (semiaxes ![]() ,
, ![]() , and
, and ![]() ). Thus, the
theory allows for a particle-shape dependence of elastic strain
energy.
). Thus, the
theory allows for a particle-shape dependence of elastic strain
energy.
Two cases: Pure dilatational transformation; pure shear transformation
 |
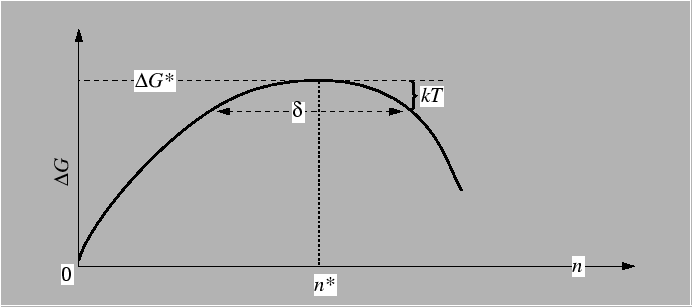
Nucleation kinetics
Equilibrium distribution of clusters. Effect of losing clusters at sizes greater than critical sizes. Concept of "diffusion equation" for steady-state distribution when clusters greater than critical size are being lost.
Key results:
Time-dependent nucleation
 |
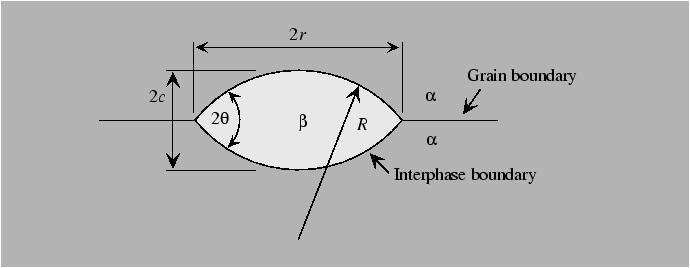
Heterogeneous nucleation
Heterogeneous nucleation can occur on imperfections when the nucleating particle effectively "replaces" some energetic feature of the original configuration. For instance, nucleation on a grain boundary removes grain boundary area and replaces it with interphase boundary area that would have been created anyway by formation of the new phase. Thus, prexisting imperfections effectively "catalyze" the nucleation process. We'll consider the case of heterogeneous nucleation on grain boundaries.
 |
Key results: