- ...-phase.1
- It may be supposed that such a
metastable composition comes about by cooling a stable composition
into a two phase field.
Such supercooling can occur because phase transformations
are retarded by the time required to form a critical nucleus.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ....2
- The volumetric
term
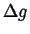 can be determined from the construction in Fig. 24-1
and is necessarily negative for nucleation (
can be determined from the construction in Fig. 24-1
and is necessarily negative for nucleation (
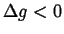 ).
Other contributions to free energy, such as stress or composition
gradients may exist, and are not
included in this simple theory.
The surface tension may also vary from absorption of solutes or the existence
of interface dislocations--these effects are also ignored here.
).
Other contributions to free energy, such as stress or composition
gradients may exist, and are not
included in this simple theory.
The surface tension may also vary from absorption of solutes or the existence
of interface dislocations--these effects are also ignored here.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ...
 3
3
-
Further reflection shows that the critical nucleus is not the one that
maximizes
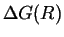 .
In the spherical example, it was assumed that the particle is always in
its most energetically favorable state.
In other words, other nuclei shapes would give larger critical nucleus volumes
for the same driving forces.
Because the shape is energy-minimizing, the critical nucleus size corresponds
to the lowest saddle point of
.
In the spherical example, it was assumed that the particle is always in
its most energetically favorable state.
In other words, other nuclei shapes would give larger critical nucleus volumes
for the same driving forces.
Because the shape is energy-minimizing, the critical nucleus size corresponds
to the lowest saddle point of 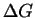 that separates subcritical nuclei from
supercritical nuclei.
that separates subcritical nuclei from
supercritical nuclei.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... so4
- This is the assumption of ``microscopic reversibility.''
It is a bit suspicious to find it in a theory of non-equilibrium processes...
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.