

Next: About this document ...
Up: Lecture_36_web
Previous: Lecture_36_web
The graphics in this section were produced by Angela Tong, MIT.
Figure:
Phase diagram for Silver-Gold is
a simple lens type.
It shows that-at least for temperatures above 900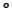 C-
the FCC phase shows complete solid solubility.
C-
the FCC phase shows complete solid solubility.
 |
Figure:
Phase diagram for light, stealthy metals,
Aluminum-Lithium.
There are five phases illustrated; however the BCC Lithium
end-member phase shows such limited Aluminum solubility that
it hardly appears on this plot.
There are two eutectics and one peritectoid reactions.
The LiAl (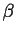 ) and
Li
) and
Li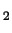 Al intermetallic phases are
ordered compounds where the atoms order on sublattices, thus changing
the symmetry of the material.
The ordered phases show limited solubility where, for instance, the
extra Li will occupy sites where an Al usually sits, or occupies
an interstitial position.
Al intermetallic phases are
ordered compounds where the atoms order on sublattices, thus changing
the symmetry of the material.
The ordered phases show limited solubility where, for instance, the
extra Li will occupy sites where an Al usually sits, or occupies
an interstitial position.
 |
Figure 36-3:
Phase diagram for Gold-Tin has seven distinct
phases, three peritectics, two eutectics, and one eutectoid reactions.
 |
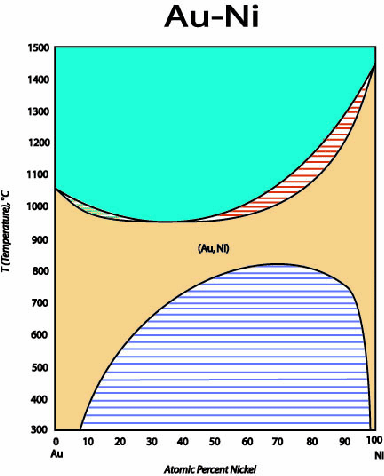
Figure:
Phase diagram for Gold-Nickel showing complete solid
solubility above about 800 C and below about 950
C and below about 950 C.
The miscibility gap at low temperatures can be understood with
a regular solution model.
C.
The miscibility gap at low temperatures can be understood with
a regular solution model.
 |


Next: About this document ...
Up: Lecture_36_web
Previous: Lecture_36_web
W. Craig Carter
2002-12-07




