To fix this idea even further,
consider a brine solution with watery-ice
and salty-water. The quantity of interest may
be
the temperature (or temperatures) that
an average composition of
![]() has an icy-phase in equilibrium with a
watery-phase. One possible state of the
system may be:
has an icy-phase in equilibrium with a
watery-phase. One possible state of the
system may be:
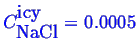 and
and
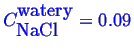
which completely determines the phase fractions.
Note that this doesn't determine what the extent
(i.e.
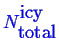 and
and
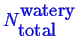 ) of the system is--it
only produces derived intensive quantities.
) of the system is--it
only produces derived intensive quantities.