


Next: Phase Diagram Construction
Up: Continuum Thermodynamics
Previous: Le Chatelier's Principle
You might have noticed that we have defined the Gibbs free energy in two different ways
above, once by subtracting off combinations of
 ,
,
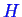 , and
, and
 and once as a sum
of chemical potentials and amounts of species. The fact that they are equal gives a
new useful relation called the ``Gibbs-Duhem Relation.'' The Gibbs-Duhem relation allows
us to calculate relationships between quantities as a system remains in equilibrium.
One example is the Clausius-Clapeyron equation that relates equilibrium changes in pressure
to changes in temperature as a function of material parameters.
These relationships restrict the degrees of freedom in a system at equilibrium. These
restrictions make up the famous ``Gibbs Phase Rule''
and once as a sum
of chemical potentials and amounts of species. The fact that they are equal gives a
new useful relation called the ``Gibbs-Duhem Relation.'' The Gibbs-Duhem relation allows
us to calculate relationships between quantities as a system remains in equilibrium.
One example is the Clausius-Clapeyron equation that relates equilibrium changes in pressure
to changes in temperature as a function of material parameters.
These relationships restrict the degrees of freedom in a system at equilibrium. These
restrictions make up the famous ``Gibbs Phase Rule''
 .
The Gibbs phase rule tells you
how many things you can change and still have equilibrium and it depends on the number
of chemical components and the number of existing phases. You will all know Gibbs phase
rule by the end of the class--I hope I can also teach you what it means.
.
The Gibbs phase rule tells you
how many things you can change and still have equilibrium and it depends on the number
of chemical components and the number of existing phases. You will all know Gibbs phase
rule by the end of the class--I hope I can also teach you what it means.



Next: Phase Diagram Construction
Up: Continuum Thermodynamics
Previous: Le Chatelier's Principle
W. Craig Carter
2002-09-05