


Next: Extrapolation to Non-Stable States
Up: Continuum Thermodynamics
Previous: Phase Changes
The molar entropies of dense objects tends to be smaller than not dense objects so
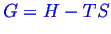 tends
to favor not-dense objects (gases) at high temperatures.
The molar entropies of complex crystals are generally larger than simple ones.
Molar entropies of ordered systems like crystals generally are generally smaller
than those with no long-range order like fluids.
Apparently, entropy has something to do with the number of different ways a system can
be rearranged and still have the same energy.
tends
to favor not-dense objects (gases) at high temperatures.
The molar entropies of complex crystals are generally larger than simple ones.
Molar entropies of ordered systems like crystals generally are generally smaller
than those with no long-range order like fluids.
Apparently, entropy has something to do with the number of different ways a system can
be rearranged and still have the same energy.
W. Craig Carter
2002-09-05