


Next: The Second Law
Up: Continuum Thermodynamics
Previous: Entropy
It is useful to imagine a system that changes at constant pressure. In this case, the
change in energy as a body is heated is not exactly the internal energy, but
a new state function called enthalpy
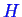 .
.
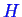 represents the available thermal energy
at constant pressure. It is the first new derived state function we will learn about after
entropy.
represents the available thermal energy
at constant pressure. It is the first new derived state function we will learn about after
entropy.
W. Craig Carter
2002-09-05